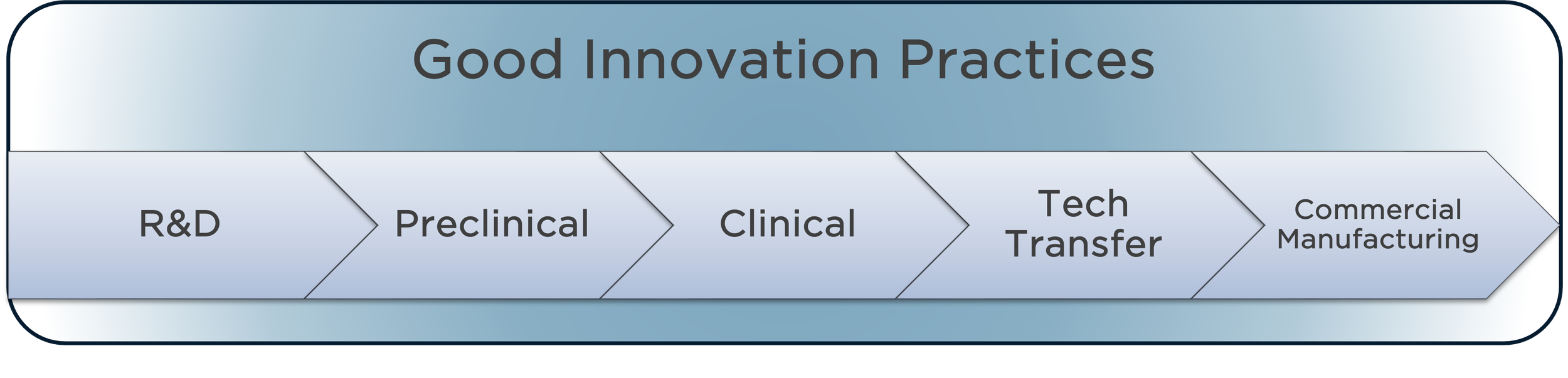
Good Innovation Practices™ (GIP) helps life science organizations go from bench to patient faster — while staying agile, compliant, and audit-ready.
Good Innovation Practices™ (GIP) is a structured, agile, and risk-based framework that provides core principles that help accelerating the product lifecycle stages from R&D to commercialization. GIP aims to:
• Align quality strategy with product maturity: phase-appropriate controls.
• Empower cross-functional teams: collaboration over silos.
• Promote “right-sized” quality: compliance without over-engineering.
What are GIP principles?
Risk-Based Quality Integration
What it means:
Tailored quality systems and controls based on product maturity and risk level.
Why it matters:
Enables right-sized compliance, avoiding over- or under-regulation while maintaining GMP alignment.
Agile, Phase-Appropriate Execution
What it means:
Implement fit-for-purpose processes that evolve with development stages.
Why it matters:
Supports flexibility and speed from R&D through tech transfer and scale-up.
Cross-Functional Collaboration
What it means:
Break down silos between QA, R&D, manufacturing, and regulatory teams.
Why it matters:
Encourages early alignment, reduces rework, and strengthens tech transfer success.
What does GIP offer?
🧪 Agile Quality Frameworks
📊 Digitall Enabled Execution Tools
🧭 GIP Compliance Navigator
Tools and insights designed to embed innovation and quality from R&D to commercialization — built on global regulatory expectations and industry best practices like ICH Q10.
Sign-up for more information.
🔒 No spam. No costs. Just smart insights on quality and innovation.